Covid-19 Diagnostic
In Collaboration with Siloam Hospital Lippo Village
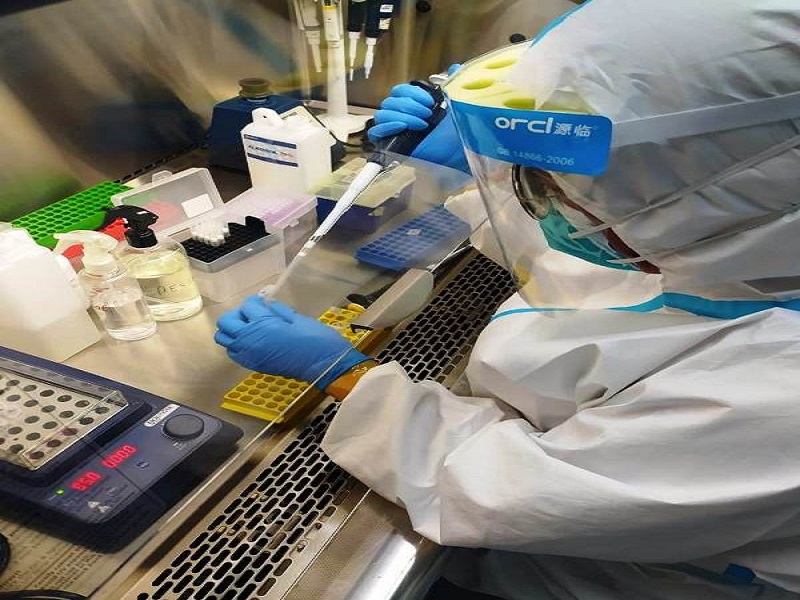
Since the pandemic outbreak, MRIN has been actively involved in the diagnosis of Covid-19. Three biosafety level (BSL) 2+ facilities have been set up to process patient samples from Siloam hospitals. Samples coming in viral transport medium (VTM) solutions are processed to isolate the viral RNA. This process requires laboratories that meet the BSL2+ regulations. During the isolation of viral RNA virus particles are destroyed, only genetic material of the virus is obtained. In the form of RNA, this biological material is no longer infectious and can then be proceeded for the detection of the SARS-CoV-2 virus in real-time Polymerase Chain Reaction (RT-PCR). For the detection of the SARS-CoV-2, RT-PCR uses two primer pairs specific for two different SARS-CoV-2 genes. As internal control, a primer pair for a specific human gene is used (this is a control for PCR reaction as well as control for RNA preparation).
Ct number values, or missing of Ct numbers, tell us whether the test was positive or negative. Ct number is the cycle threshold number at which the products amplified in the RT-PCR appears or detectable. If the result is not clear, reactions may to be repeated. The results obtained from the PCR machines in the MRIN laboratories are then forwarded to the diagnostic laboratories of the Lippo Village Siloam Hospital for validation before release to the patients.
MRIN-Siloam covid-19 laboratory has high standard operating procedures and has been set up to meet the ministry of health's requirements as stated in the Minister of Health's circular number HK.02.01/Menkes/234/2020 regarding laboratories guidelines for performing RT-PCR tests to diagnose covid-19. Our laboratory has so far been testing approx. 150,000 samples with a maximum test capacity of 960 samples per day (status February 2021).
RT-PCR machines for SARS-CoV2 detection
BSL 2 + facilities for isolation of viral RNA