Our Services
MRIN-ACRO offers a range of preclinical drug
development services including drug services. Our expertise spans across rodent toxicology, pharmacodynamics
study, and method consultation and validation. Our
multidisciplinary research team led by experts has helped our clients in
successful project submissions.
MRIN-ACRO offers a range of preclinical drug development services including drug services. Our expertise spans across rodent toxicology, pharmacodynamics study, and method consultation and validation. Our multidisciplinary research team led by experts has helped our clients in successful project submissions.
Toxicology Studies in
Rodent:
1.General
toxicology studies (toxicology acute oral, toxicology sub chronicoral, and
chronic toxicology oral studies)
2.Single-dose
studies
3.Repeated-dose
studies
4.Reference:
Toxicology Studies in
Rodent:
1.General
toxicology studies (toxicology acute oral, toxicology sub chronicoral, and
chronic toxicology oral studies)
2.Single-dose
studies
3.Repeated-dose
studies
4.Reference:
1.General toxicology studies (toxicology acute oral, toxicology sub chronicoral, and chronic toxicology oral studies)
2.Single-dose studies
3.Repeated-dose studies
4.Reference:
A. Peraturan Kepala
BPOM Nomor 10 tahun 2022 Tentang Uji Toksisitas Praklinik Secara In Vivo (The Regulation
of Head of Drug and Food Control Agency Number 10 Year 2022 on In Vivo
Preclinical Toxicology Testing) (Link BPOM)
B. Peraturan Kepala
BPOM Nomor 7 tahun 2014 tentangPedoman Uji Toksisitas Nonklinik Secara In Vivo (The
Regulatio of Head of Drug and Food Control Agency Number 7 Year 2014 on In vivo
Nonclinical Toxicology Testing Protocols) (Link BPOM)
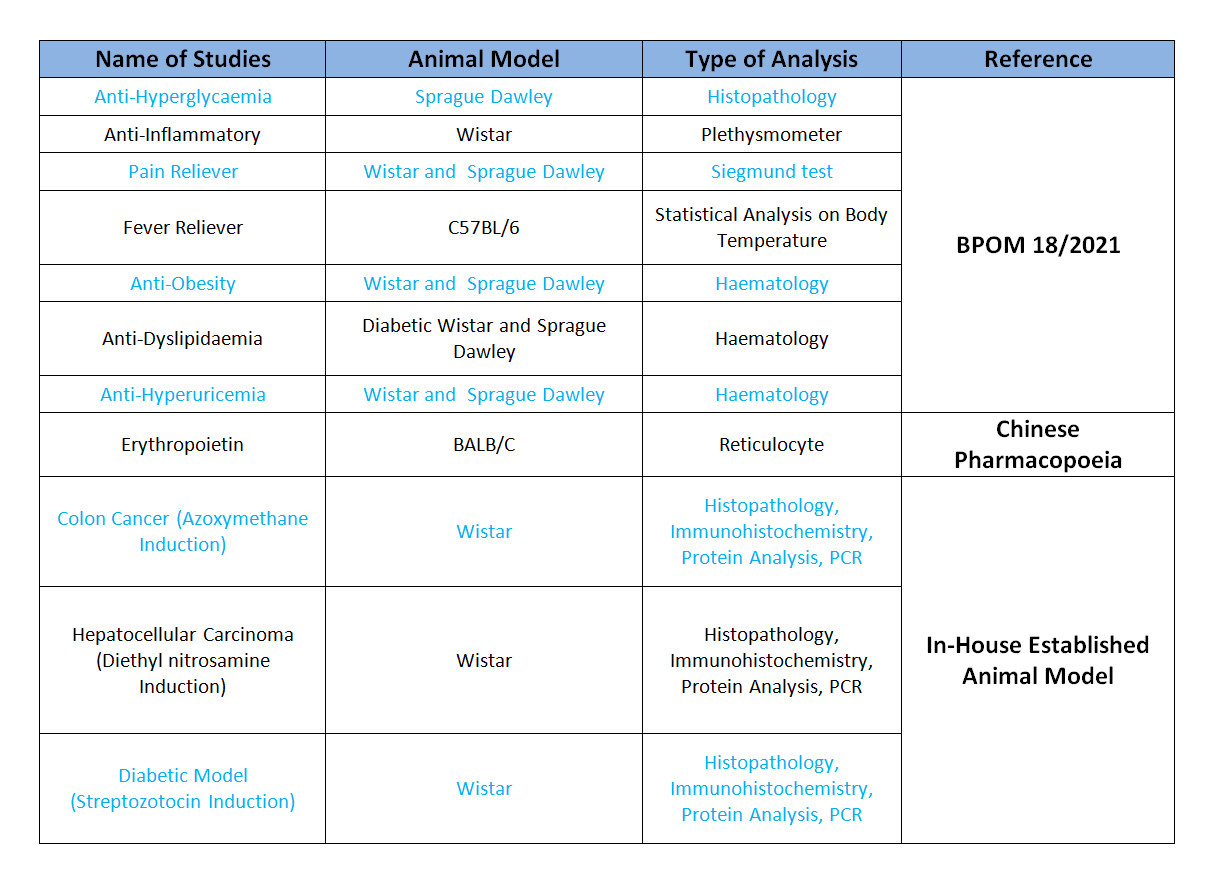
Pharmacodynamics Studies:
Pharmacodynamics Studies:
Biopharmaceuticals Services.
MRIN-ACRO is committed to provide
biopharmaceuticals services to support preclinical studies, and development of
vaccine and other novel drug products.
Our services include:
1.Vaccine
Immunogenicity
2.Cell
cultures based in vitro test system
3.Molecular
Biology analysis
4.Specimen
Storage

